|
Ordering information |
|||
|
Name |
Cat. No. |
Vol. |
Scheme |
|
G-DBCO |
PMG035-2 |
2 ml |
|
1. Overview
Click Chemistry describes rapid, selective "click" reactions between paired functional groups in mild aqueous solutions. This concept has evolved into a convenient, versatile, and reliable two-step coupling procedure for Molecules A and B, widely applied in biosciences, drug discovery, and materials science.
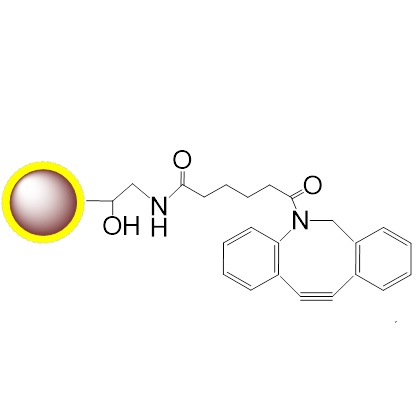
PuriMag? G-DBCO, Magnetic Beads feature DBCO (Dibenzocyclooctyne) covalently coupled to superparamagnetic nanoparticles. These beads enable rapid, efficient, and spontaneous catalyst-free CLICK chemistry under physiological conditions to capture azide-tagged biomolecules for:
Labeling & Tracking
Purification & Isolation
Protein-Protein/Biomolecule/DNA-Protein interaction studies
Synonyms: DBCO = ADIBO (Azadibenzocyclooctyne) = DIBAC (Dibenzoazacyclooctyne)
Key Mechanism: Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC)
Bioorthogonal reaction between DBCO and azides without copper catalysts
Minimal off-target labeling in cells/tissue lysates
Preserves biological integrity during conjugation
Advantages of Click Chemistry:
High specificity & low background: Inert to native functional groups (e.g., amines)
Rapid quantitative labeling: Enables non-radioactive analysis of enzymatic activity in vitro/vivo
Small molecular footprint: Excellent substrate properties
Features of DBCO Beads:
Covalent capture without cytotoxic catalysts
Ultra-low non-specific binding after stringent washing
Compatibility with complex biological matrices
Schematic Diagram of PuriMag? Bead Coupling Mechanism:

2. product description
Product Specifications
Description
Polymer coated Fe3O4 nanoparticles
Particle Size
200 nm
Number of Beads
~1.7×1010 beads/mg
Matrix
Proprietary polymer
Functional group
DBCO group
Group density
~100 μmole / g of Beads
Magnetization
60~70 EMU/g
Formulation
10mg/mL in DI water
Storage
1 year at 2~8 ℃. Do not freeze.
3. Instructions for Use
1.Mix ~1 mg DBCO Magnetic Nanoparticles with 60 μM azide-tagged ligand in 100 μL PBS buffer (pH 7.0).
2.React at 30°C for 2 h with gentle agitation.
3.Recover the product via magnetic separation.
4.Wash 3× with PBS (pH 7.0).
(For research use only!)